Total Dissolved Solids - TDS
Total Dissolved Solids (TDS) is a measure of the combined content of all contaminants contained in drinking water.
A standard definition for “dissolved solids” is that they must be small enough to pass through a 2 micron filter. Contaminants larger than 2 microns are often referred to as Total Suspended Solids.
Total Dissolved Solids are classified by the EPA as a Secondary Contaminant. As a whole, they are considered more of a nuisance than a threat. Certain individual water contaminants that contribute to the Total Dissolved level, however, may pose long term health risks if they exceed certain levels.
Where Do Total Dissolved Solids Come From?
Minnesota drinking water sources include rivers, lakes, reservoirs, springs, and wells.
As water travels over the surface of the land or through the ground, it dissolves naturally occurring minerals and, in some cases, radioactive material.
Water can also pick up substances from animals or human activity like industry and agriculture.
Contaminants that may be present in source water include:
Inorganic Contaminants: Salts and metals can be naturally‐occurring or result from urban storm water runoff, industrial or domestic wastewater discharges, oil and gas production, mining or farming.
Organic Contaminants: Synthetic and volatile organic chemicals are by‐products of industrial processes and petroleum production, and can also come from gas stations, urban storm water runoff, and septic systems.
Disinfectants and Disinfection Byproducts: When disinfection chemicals like chlorine are added by water treatment plants to control bacteria, they may interact with organic matter to create a new class of chemicals called “Disinfection Byproducts”.
Radioactive contaminants: Can be naturally‐occurring or the result of oil and gas production and mining activities.
Microbial Contaminants: Viruses and bacteria may come from sewage treatment plants, septic systems, agricultural livestock operations, and wildlife.
How are Total Dissolved Solids Regulated?
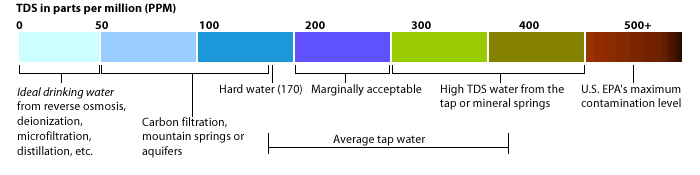
The EPA Secondary Regulations advise a maximum contamination level (MCL) of 500mg/liter, or 500 parts per million (ppm) for TDS. Numerous water supplies exceed this level. When TDS levels exceed 1000mg/L it is generally considered unfit for human consumption.
A high level of TDS is an indicator of potential concerns, and warrants further investigation. Most often, high levels of TDS are caused by the presence of potassium, chlorides and sodium. These ions have little or no short-term effects, but toxic ions (Arsenic, Chromium, Manganese, Nitrates and others) may also be dissolved in the water.
How are Total Dissolved Solids Measured?
A TDS meter is based on the electrical conductivity (EC) of water. Pure water has virtually zero conductivity.
Conductivity is usually about 100 times the total cations or anions expressed as equivalents. TDS is calculated by converting the EC by a factor of 0.5 to 1.0 times the EC, depending upon the levels. Typically, the higher the level of EC, the higher the conversion factor to determine the TDS.
NOTE – While a TDS meter is based on conductivity, TDS and conductivity are not the same thing.
Can Total Dissolved Solids Affect Health?
Water that is high in TDS may taste bitter, salty, or metallic and may have unpleasant odors.
High TDS water is less thirst quenching, and can interfere with the taste of foods and beverages, and makes them less desirable to consume. In the book “Water Can Undermine Your Health, Norm Walker states:
“If a person drinks 2 pints of water a day, this will total 4,500 gallons of water passing through his body over a 70 year span.
If the water is not totally pure, this 4,500 gallons will include 200-300 pounds of rock that the body cannot utilize. Most will be eliminated through excretory channels.
But some of this will stay in the body, causing stiffness in the joints, hardening of the arteries, kidney stones, gall stones and blockages of arteries, microscopic capillaries and other passages in which liquids flow through our entire body.”

Total Dissolved Solids Increase Water Heater Corrosion
Many water heater manufacturers place a limit of 500ppm TDS in their warranty.
Salts and metals impart a slight conductivity to water. Through an electrical process called electrolysis, this conductivity will eventually cause metal to rust or corrode. When water is heated, this electrical process can be accelerated.
Most water heaters are made of a steel tank with a porcelain enamel (glass) lining.
It is not always possible to completely cover the inside of the tank, and it’s important to provide metal that can be consumed by the electrical process.
This is where the sacrificial anode rod comes in. By acting as a lightning rod for the corrosion process, the anode rod draws the harmful electrolytic process away from the water heater tank and focuses the corrosion on the anode rod.
Hard Water or soft water that is high in TDS need this sacrificial anode rod to ensure that the electrolysis doesn’t artificially shorten the life of the water heater.
What Treatment Systems Remove Total Dissolved Solids?
There are three proven technologies for reducing TDS:
Reverse Osmosis (RO) remove TDS by forcing the water, under pressure, through a synthetic membrane. The membrane contains microscopic pores which will allow only molecules smaller than 0.0001 micron to pass through. Since the molecules of dissolved metals and salts are large compared to the water molecules, the water will squeeze through the membrane leaving the metals and salts behind.
A professional Reverse Osmosis system is capable of removing 90-99% of the dissolved mineral salts from water. A pre-filter is usually required to protect the membrane and remove organics.
The HERO System is a type of whole business or whole house reverse osmosis that is used as both a salt-free water softener, and a purifier. Whole house systems like the HERO have the added benefit of protecting water heaters from corrosion, in addition to high quality drinking water.
Distillers are better known as “stills.” Stills work by heating small amounts (less than 2 gallons) of water to produce steam. The steam is then collected and condensed back into water. The dissolved mineral salts will not vaporize and are left behind in the heating chamber.
Stills require frequent, rigorous cleaning to remove the baked-on mineral salts. They also produce a “flat” taste and acidic water which has kept them from becoming a popular drinking water solution.
Deionization (DI) Systems pass water through a resin cartridge that attracts the dissolved solids, producing a high-purity water. Because DI cartridges have a limited life and require frequent replacement they are generally not used for high volume applications.
