Perchlorate in Minnesota Drinking Water

Perchlorate is used in a wide range of applications, including military munitions (mortars, flares, grenades), solid rocket fuel, pyrotechnics and fireworks, blasting agents, matches, air bags, and certain types of fertilizers.
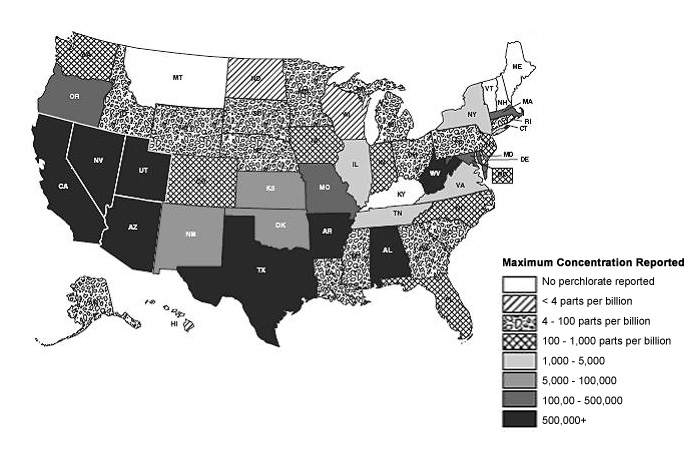
The EPA has estimated that Perchlorate contamination occurs in drinking water sources in 22-35 US states with up to 17 million people consuming water with greater than 4 parts per billion (ppb).
Different Types of Perchlorate in Water
Much of the Perchlorate discussed in the news is of the man-made variety. But Perchlorate can naturally occur in soils with high concentrations of organic salts that convert to Perchlorate in the presence of sunlight and/or ultraviolet light. Common salts include:
- Ammonium Perchlorate (NH4ClO4)
- Potassium Perchlorate (KClO4)
- Magnesium Perchlorate (MgClO4)
- Sodium Perchlorate (NaClO4)
These salts pose a great threat to drinking water as the Perchlorate ion (ClO4) is very stable once it dissolves into water.
Health Effects of Perchlorate Water Contamination
Perchlorate prevents iodide uptake into the Thyroid Gland and can reduce its ability to produce enough hormone. Tumors may also develop as a result of changes in the thyroid hormone levels.
Expectant mothers, developing fetuses and infants are at greatest risk for exposure to Perchlorate contamination.
Standards for Perchlorate in Water
In February 2011, the EPA moved to regulate Perchlorate under the Safe Drinking Water Act (SDWA). The EPA has suggested a 1 ppb level maximum in public water supplies. Many Perchlorate plumes in the United States, including the Colorado River, are believed to be in the range between 4-100ppb.
The most accurate testing method is EPA Method 314 that uses Ion Chromatography (IC), where an aqueous sample is filtered through a 0.45 micron filter and then introduced into the IC. The Perchlorate is then detected by a conductivity detector.
Effective Water Treatment Methods for Perchlorate
Health experts say that Perchlorate cannot be ingested through the skin, so the main emphasis for water treatment should be for drinking water and food preparation.
Water treatment for municipalities or water utilities is very complicated because the Perchlorate ion does not respond to classic techniques such as sand filtration or coagulation. It can persist for years in the environment because it does not react with other compounds present in the water.
There are currently two proven treatment technologies available:
Reverse Osmosis
Under-sink Reverse Osmosis Systems, and whole-house/business purification systems like the Pureoflow, can provide about 95% rejection of Perchlorates. For extremely high levels, a specialty Ion Exchange polishing filter might be required after the RO to achieve very low levels.
Anion Exchange
There are also specialty Anion Exchange resins that have very strong affinities to the Perchlorate ion. They can be used on a disposable basis, or they can be regenerated for a longer service life. The LINX Drinking Water System uses electricity to regenerate a special film made of Anion resin. This allows the system to effectively remove Perchlorate, without salt or chemicals required by other resins.
